We ensure compliance to international standards
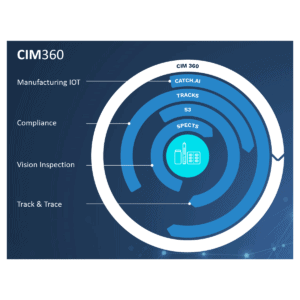
CIM delivers plug-and-play technology in the pharmaceutical industry. Our solutions and products are compatible with any pre-existing system, and compliance to all international standards is guaranteed. We ensure minimal impact on OEE with our module-based, easy-to-install, and easy-to-use solutions and product.
We call it CIM 360
Reap the fruits of your hidden data
CATCH.AI is industrial IoT software that collects data from your production set-up and optimizes in places you didn’t even know existed. CATCH.AI also enables machine learning, so that you can get the most out of your production.
Discover new ways to optimize
CATCH.AI analyses the data streams in your operation and reveals new ways for you to optimize. Instead of just using predetermined KPI’s to evaluate the performance of your operation, CATCH.AI gives you answers to questions that you didn’t know you should ask.
- Optimizes your equipment efficiency
- Enables predictive maintenance and machine learning
- Covers all parts of the data flow
- Integrates with any existing components or systems
- User-friendly and customized dashboard
Keep Track and Stay Compliant
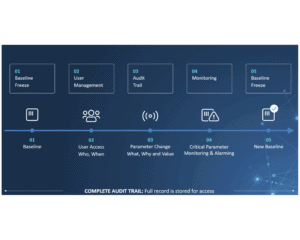
For production companies, keeping track of the activities on assembly lines and packaging lines is key to staying compliant with international standards and regulations. This is especially true for the pharma industry, where the CFR 21-part 11 regulation sets high standards for electronic documentation and signatures. Failure to comply means failure to operate, and that is devastating to business. So choosing the right audit trail system is more important than ever.
CIM TRACKS acts as a smart bridge between machine components at level 1 and the Enterprise System at level3 and allows you to monitor critical parameters and perform user management much more efficiently.
Your benefits
Choosing CIM SPECTS gives you a number of benefits:
- Compliance to international standards is guaranteed
- Plug-and-play technology for easy integration
- Minimal impact on OEE
- CIM is your dedicated partner all the way
Everything included
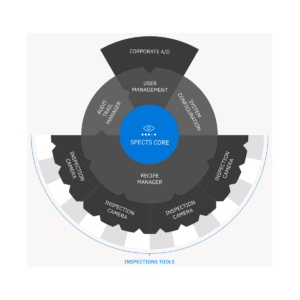
A CIM SPECTS solution is customized to match your needs completely, but it always includes:
- User Management system with integration to corporate domain and AD
- Trail system that meets audits from regulatory authorities
- CFR21 part 11 compliance readiness
- Flexible recipe-based inspections
- Intuitive user interfaces
- Full documentation package
- Industrial grade vision components
Serialization for pharma
Our serialization solutions guarantee full pharmaceutical traceability and integrate with any existing site server, line control or inspection system. Our solutions are based on pre-defined modules, and the need for customization is minimal. The result is a plug-and-play system that enables pharmaceutical companies to comply with all international serialization standards and distribute their products globally.
Get everything printed on the production line
Integrate printing into your production line for reduced batch change-over-time, maximum flexibility, and improved quality control.
- From print file to Inspection
- Full Content Printing
- Full Content Inspection (Color, OCV, Defects)
Minimize Batch Change over time, Line Clearance and Line Validation
References
Don't take our word for it
Henrik Just
Project Manager Serialization
Stevanato Group
Jan Bigum
Lead Engineer
Grundfos Production IT
Andy Cumming
Consultant in Manufacture Change


Contact our specialist in Pharma Solutions
Anders Meister, Chief Commercial Officer